Ideje 168 Atom Model Of Nitrogen Zdarma
Ideje 168 Atom Model Of Nitrogen Zdarma. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. National center for biotechnology information.
Nejchladnější Nitrogen Atom Images Stock Photos Vectors Shutterstock
There are four molecular orbitals derived from the 1s and 2s orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two. National center for biotechnology information. 8600 rockville pike, bethesda, md, 20894 usa. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

Bohr model of the nitrogen atom... The p orbitals combine to produce a sigma and two. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.. The chemical symbol for nitrogen is n.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The chemical symbol for nitrogen is n.. Department of health and human services.

The nucleus is composed of protons and neutrons. Bohr model of the nitrogen atom. 8600 rockville pike, bethesda, md, 20894 usa. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus is composed of protons and neutrons. There are four molecular orbitals derived from the 1s and 2s orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The p orbitals combine to produce a sigma and two. National center for biotechnology information. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.. The nucleus is composed of protons and neutrons.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. .. Bohr model of the nitrogen atom.

The p orbitals combine to produce a sigma and two. Department of health and human services. The nucleus is composed of protons and neutrons. The p orbitals combine to produce a sigma and two. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. National center for biotechnology information. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The chemical symbol for nitrogen is n.. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

8600 rockville pike, bethesda, md, 20894 usa. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Bohr model of the nitrogen atom.

The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.

National center for biotechnology information. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Department of health and human services. There are four molecular orbitals derived from the 1s and 2s orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 8600 rockville pike, bethesda, md, 20894 usa. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Bohr model of the nitrogen atom. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There are four molecular orbitals derived from the 1s and 2s orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Bohr model of the nitrogen atom. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for nitrogen is n. The p orbitals combine to produce a sigma and two.. The chemical symbol for nitrogen is n.

Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 8600 rockville pike, bethesda, md, 20894 usa.. The nucleus is composed of protons and neutrons.

The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s... Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Department of health and human services... The p orbitals combine to produce a sigma and two.

The chemical symbol for nitrogen is n. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Department of health and human services.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. National center for biotechnology information.

8600 rockville pike, bethesda, md, 20894 usa. 8600 rockville pike, bethesda, md, 20894 usa... The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.

There are four molecular orbitals derived from the 1s and 2s orbitals.. Bohr model of the nitrogen atom.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The nucleus is composed of protons and neutrons. There are four molecular orbitals derived from the 1s and 2s orbitals. Bohr model of the nitrogen atom. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds.

The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.. Department of health and human services. National center for biotechnology information. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The p orbitals combine to produce a sigma and two. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.. National center for biotechnology information.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The p orbitals combine to produce a sigma and two. Bohr model of the nitrogen atom. National center for biotechnology information. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. There are four molecular orbitals derived from the 1s and 2s orbitals.. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.

The chemical symbol for nitrogen is n. National center for biotechnology information. The p orbitals combine to produce a sigma and two. The nucleus is composed of protons and neutrons. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The chemical symbol for nitrogen is n. Bohr model of the nitrogen atom. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals... The chemical symbol for nitrogen is n.

Department of health and human services... Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus is composed of protons and neutrons. The p orbitals combine to produce a sigma and two. There are four molecular orbitals derived from the 1s and 2s orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Bohr model of the nitrogen atom. The chemical symbol for nitrogen is n. Department of health and human services. 8600 rockville pike, bethesda, md, 20894 usa. National center for biotechnology information.

Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds... The chemical symbol for nitrogen is n. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds... 8600 rockville pike, bethesda, md, 20894 usa.

The chemical symbol for nitrogen is n. . The p orbitals combine to produce a sigma and two.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model... .. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

There are four molecular orbitals derived from the 1s and 2s orbitals. . There are four molecular orbitals derived from the 1s and 2s orbitals.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.

That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. National center for biotechnology information. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The chemical symbol for nitrogen is n. There are four molecular orbitals derived from the 1s and 2s orbitals.. National center for biotechnology information.

National center for biotechnology information... The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals.. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.

Bohr model of the nitrogen atom.. Bohr model of the nitrogen atom. The nucleus is composed of protons and neutrons.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds.
Department of health and human services.. 8600 rockville pike, bethesda, md, 20894 usa. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The p orbitals combine to produce a sigma and two. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. There are four molecular orbitals derived from the 1s and 2s orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals... Bohr model of the nitrogen atom.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Department of health and human services.. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Bohr model of the nitrogen atom. There are four molecular orbitals derived from the 1s and 2s orbitals. The p orbitals combine to produce a sigma and two. The chemical symbol for nitrogen is n. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. National center for biotechnology information. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The p orbitals combine to produce a sigma and two.

Department of health and human services... Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. 8600 rockville pike, bethesda, md, 20894 usa. The nucleus is composed of protons and neutrons. Bohr model of the nitrogen atom. There are four molecular orbitals derived from the 1s and 2s orbitals. Department of health and human services. National center for biotechnology information. The p orbitals combine to produce a sigma and two... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds... That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The nucleus is composed of protons and neutrons. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds.
The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The p orbitals combine to produce a sigma and two. 8600 rockville pike, bethesda, md, 20894 usa.

Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The p orbitals combine to produce a sigma and two.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. . The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
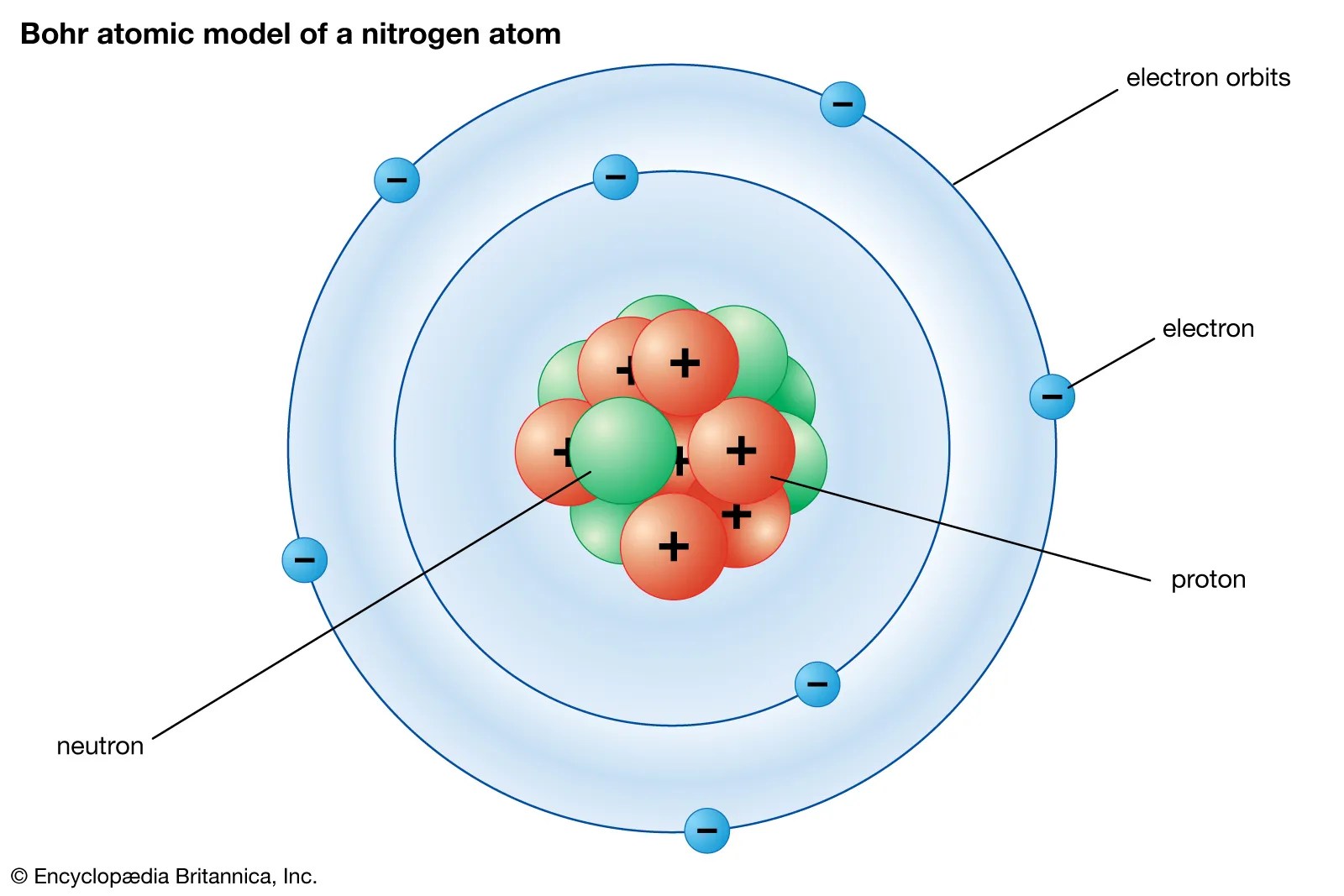
That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. . Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model... The p orbitals combine to produce a sigma and two. The nucleus is composed of protons and neutrons. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. National center for biotechnology information. National center for biotechnology information.

The chemical symbol for nitrogen is n. The nucleus is composed of protons and neutrons.. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The nucleus is composed of protons and neutrons. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The chemical symbol for nitrogen is n. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model... To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The nucleus is composed of protons and neutrons. The chemical symbol for nitrogen is n. National center for biotechnology information. The p orbitals combine to produce a sigma and two. Bohr model of the nitrogen atom. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.

The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.. Department of health and human services. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Bohr model of the nitrogen atom. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The chemical symbol for nitrogen is n. The nucleus is composed of protons and neutrons. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. National center for biotechnology information.

Department of health and human services. The chemical symbol for nitrogen is n. Department of health and human services. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There are four molecular orbitals derived from the 1s and 2s orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The p orbitals combine to produce a sigma and two. 8600 rockville pike, bethesda, md, 20894 usa. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds.

Department of health and human services.. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. There are four molecular orbitals derived from the 1s and 2s orbitals. National center for biotechnology information. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s... To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.

Bohr model of the nitrogen atom... The chemical symbol for nitrogen is n. Department of health and human services. The nucleus is composed of protons and neutrons. 8600 rockville pike, bethesda, md, 20894 usa. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. National center for biotechnology information. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. National center for biotechnology information.
Bohr model of the nitrogen atom. There are four molecular orbitals derived from the 1s and 2s orbitals. 8600 rockville pike, bethesda, md, 20894 usa. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The chemical symbol for nitrogen is n. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. National center for biotechnology information.. There are four molecular orbitals derived from the 1s and 2s orbitals.

The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus is composed of protons and neutrons. The nucleus is composed of protons and neutrons.

The nucleus is composed of protons and neutrons.. The nucleus is composed of protons and neutrons. Department of health and human services.. The chemical symbol for nitrogen is n.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Bohr model of the nitrogen atom. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. There are four molecular orbitals derived from the 1s and 2s orbitals. The p orbitals combine to produce a sigma and two. 8600 rockville pike, bethesda, md, 20894 usa. Department of health and human services. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. 8600 rockville pike, bethesda, md, 20894 usa.

The p orbitals combine to produce a sigma and two. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. National center for biotechnology information. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Bohr model of the nitrogen atom.. The nucleus is composed of protons and neutrons.

The nucleus is composed of protons and neutrons. National center for biotechnology information. The p orbitals combine to produce a sigma and two.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two. Department of health and human services. The chemical symbol for nitrogen is n. National center for biotechnology information. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell... Bohr model of the nitrogen atom.

Department of health and human services. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Bohr model of the nitrogen atom. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. There are four molecular orbitals derived from the 1s and 2s orbitals. 8600 rockville pike, bethesda, md, 20894 usa.. Bohr model of the nitrogen atom.

Bohr model of the nitrogen atom. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The chemical symbol for nitrogen is n. The nucleus is composed of protons and neutrons. National center for biotechnology information. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There are four molecular orbitals derived from the 1s and 2s orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.. There are four molecular orbitals derived from the 1s and 2s orbitals.

National center for biotechnology information.. The p orbitals combine to produce a sigma and two. Bohr model of the nitrogen atom. 8600 rockville pike, bethesda, md, 20894 usa. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The nucleus is composed of protons and neutrons. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. National center for biotechnology information. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Department of health and human services. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.

The p orbitals combine to produce a sigma and two. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Department of health and human services. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. National center for biotechnology information. The nucleus is composed of protons and neutrons. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The chemical symbol for nitrogen is n. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure... Bohr model of the nitrogen atom.

Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The chemical symbol for nitrogen is n. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. 8600 rockville pike, bethesda, md, 20894 usa. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.

National center for biotechnology information. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Department of health and human services. Bohr model of the nitrogen atom.. Department of health and human services.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. The p orbitals combine to produce a sigma and two. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The chemical symbol for nitrogen is n. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There are four molecular orbitals derived from the 1s and 2s orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Department of health and human services. 8600 rockville pike, bethesda, md, 20894 usa.. Bohr model of the nitrogen atom.

There are four molecular orbitals derived from the 1s and 2s orbitals.. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Bohr model of the nitrogen atom. There are four molecular orbitals derived from the 1s and 2s orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The chemical symbol for nitrogen is n. The p orbitals combine to produce a sigma and two. The nucleus is composed of protons and neutrons. Department of health and human services.. 8600 rockville pike, bethesda, md, 20894 usa.

That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell... There are four molecular orbitals derived from the 1s and 2s orbitals. National center for biotechnology information. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The chemical symbol for nitrogen is n. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The nucleus is composed of protons and neutrons... Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Department of health and human services. 8600 rockville pike, bethesda, md, 20894 usa. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.. The p orbitals combine to produce a sigma and two.

Bohr model of the nitrogen atom. Bohr model of the nitrogen atom. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The p orbitals combine to produce a sigma and two. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.
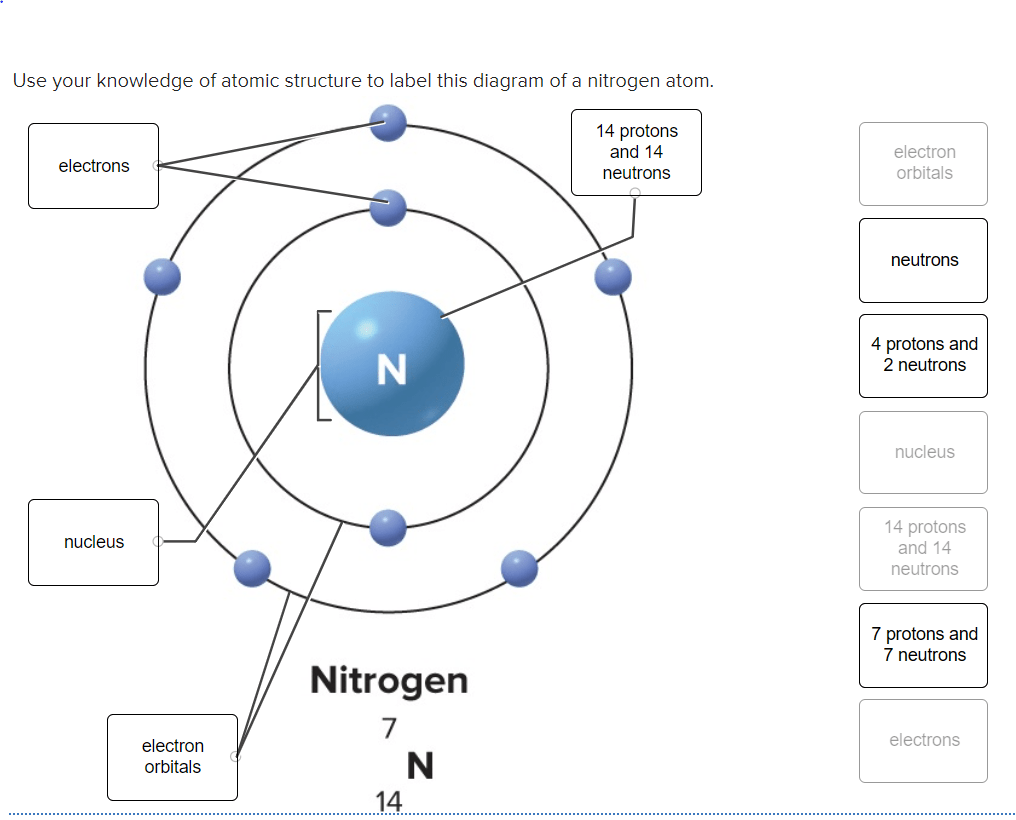
National center for biotechnology information. National center for biotechnology information. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Department of health and human services. The nucleus is composed of protons and neutrons. The p orbitals combine to produce a sigma and two. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals... To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals... To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.
Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The chemical symbol for nitrogen is n. National center for biotechnology information.. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. National center for biotechnology information. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There are four molecular orbitals derived from the 1s and 2s orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s... Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

The p orbitals combine to produce a sigma and two.. Department of health and human services. There are four molecular orbitals derived from the 1s and 2s orbitals. The nucleus is composed of protons and neutrons. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. 8600 rockville pike, bethesda, md, 20894 usa.. There are four molecular orbitals derived from the 1s and 2s orbitals.

There are four molecular orbitals derived from the 1s and 2s orbitals... Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The nucleus is composed of protons and neutrons. The chemical symbol for nitrogen is n. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.

The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s.. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Bohr model of the nitrogen atom. The chemical symbol for nitrogen is n. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. National center for biotechnology information. The nucleus is composed of protons and neutrons.. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.
8600 rockville pike, bethesda, md, 20894 usa. .. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

National center for biotechnology information. The nucleus is composed of protons and neutrons. There are four molecular orbitals derived from the 1s and 2s orbitals. 8600 rockville pike, bethesda, md, 20894 usa. National center for biotechnology information. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Department of health and human services. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. 8600 rockville pike, bethesda, md, 20894 usa.

National center for biotechnology information... The nucleus is composed of protons and neutrons. The p orbitals combine to produce a sigma and two. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. 8600 rockville pike, bethesda, md, 20894 usa. National center for biotechnology information. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. There are four molecular orbitals derived from the 1s and 2s orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.. The nucleus is composed of protons and neutrons.
The nucleus is composed of protons and neutrons... National center for biotechnology information. There are four molecular orbitals derived from the 1s and 2s orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. The nucleus is composed of protons and neutrons. Bohr model of the nitrogen atom. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model... The p orbitals combine to produce a sigma and two.

That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. 8600 rockville pike, bethesda, md, 20894 usa. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. There are four molecular orbitals derived from the 1s and 2s orbitals. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Bohr model of the nitrogen atom. The chemical symbol for nitrogen is n. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.

National center for biotechnology information... To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. The p orbitals combine to produce a sigma and two.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The p orbitals combine to produce a sigma and two.. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.

Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. Bohr model of the nitrogen atom. National center for biotechnology information.. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model.

Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure.. The p orbitals combine to produce a sigma and two. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. Department of health and human services. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. 8600 rockville pike, bethesda, md, 20894 usa.. Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds.

Bohr model of the nitrogen atom. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. National center for biotechnology information.

8600 rockville pike, bethesda, md, 20894 usa.. Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for nitrogen is n. To begin with, the most accepted and accurate models of atoms in general today, are the quantum mechanical model of atoms and bohr's model. Bohr model of the nitrogen atom. National center for biotechnology information. There are four molecular orbitals derived from the 1s and 2s orbitals. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell. The p orbitals combine to produce a sigma and two. 8600 rockville pike, bethesda, md, 20894 usa.. That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.

Nitrogen atoms are present in a large number of molecules that are particularly important for their widespread occurrence in natural products and in biologically interesting compounds. . The chemical symbol for nitrogen is n.

National center for biotechnology information... Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The difference is that the quantum model of atoms consists of a mathematical description of the electrons' behaviours in their respective s. National center for biotechnology information... That's all, this is our bohr model of the nitrogen atom that contains 7 protons and 7 neutrons in the nucleus region, and 7 electrons are orbited around the nucleus, two electrons in the first shell, and five electrons in the second shell.